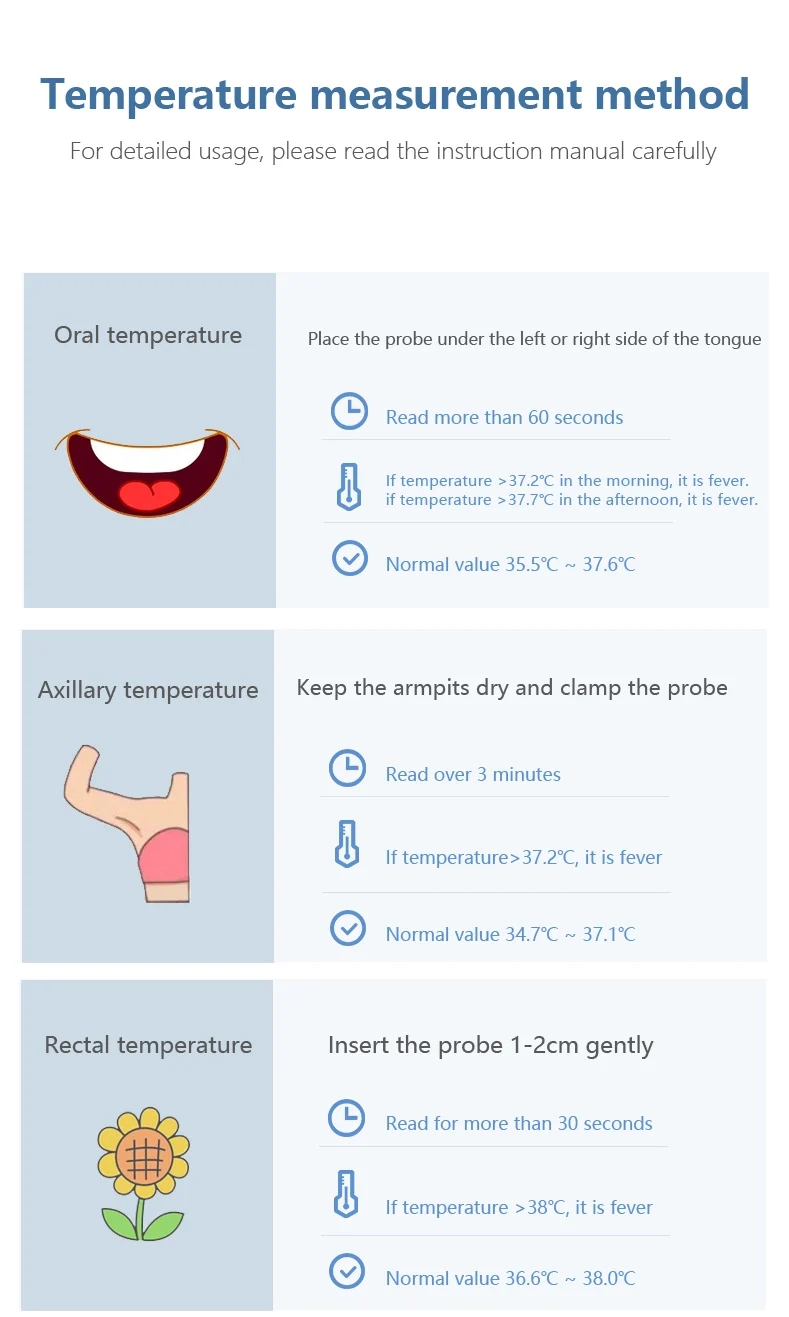
The mercury thermometer, a pivotal invention in the history of temperature measurement, was introduced in the early 18th century. This thermometer operates on the principle of mercury’s expansion and contraction within a sealed glass tube in response to temperature changes.
It consists of a glass tube with a bulb at one end and a narrow capillary tube. The bulb and part of the capillary are filled with mercury, and the rest of the tube is a vacuum or filled with an inert gas. Mercury was chosen for its unique properties: it remains liquid over a broad temperature range and expands uniformly with temperature variations.
The functioning is based on the expansion and contraction of mercury. As temperatures rise, the mercury inside the bulb and tube expands, moving up the capillary. Conversely, as temperatures drop, the mercury contracts, moving downwards. The scale alongside the tube allows for the measurement of temperature based on the height of the mercury within the calibrated markings.
This device revolutionized temperature measurement, offering precise and consistent readings compared to previous instruments. Its accuracy and ease of use made it a standard tool in various fields, including science, medicine, meteorology, and industry. However, due to environmental concerns related to mercury’s toxicity, digital thermometers and other safer alternatives have largely replaced mercury